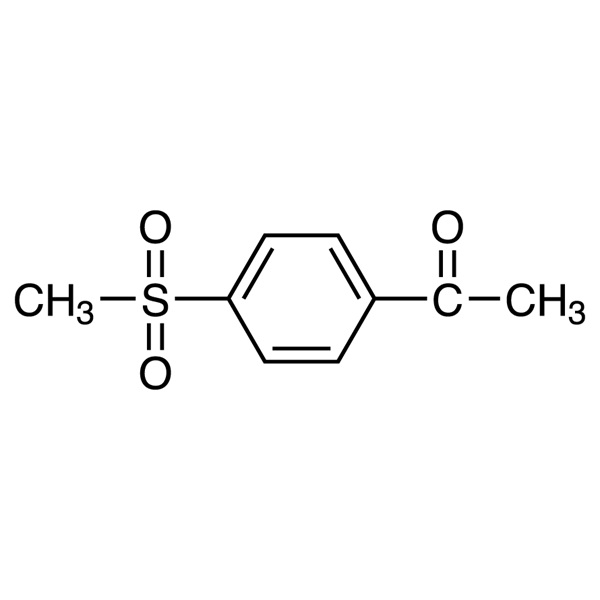
4′-(Methylsulfonyl)acetophenone CAS 10297-73-1 Purity >99.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 4'-(Methylsulfonyl)acetophenone (CAS: 10297-73-1) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | 4'-(Methylsulfonyl)acetophenone |
| Synonyms | 4-(Methylsulfonyl)acetophenone; 4-Methylsulphonylacetophenone; 4-Methyi Sulfonyl Acetophenone; 4-(Methyl Sulfonyl) Acetophenone; 1-(4-(Methylsufonyl)phenyl)ethanone; 1-[4-(Methylsulfonyl)phenyl]ethan-1-one; p-Acetylphenyl Methyl Sulfone; p-Methylsulfonylacetophenone; Etoricoxib Impurity A (USP Related Compound A) |
| CAS Number | 10297-73-1 |
| CAT Number | RF2932 |
| Stock Status | In Stock, Production Capacity 20 Tons per Month |
| Molecular Formula | C9H10O3S |
| Molecular Weight | 198.24 |
| Melting Point | 126.0~129.0℃(lit.) |
| Hazard Class | Irritant |
| HS Code | 29144000 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Light Yellow Crystal |
| Purity / Analysis Method | >99.0% (GC) |
| Melting Point | 126.0~129.0℃ |
| Water (by Karl Fischer) | <0.50% |
| Total Impurities | <1.00% |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
4'-(Methylsulfonyl)acetophenone (CAS: 10297-73-1) is an important raw material and intermediate used in organic synthesis, pharmaceuticals. 4'-(Methylsulfonyl)acetophenone is an impurity of Etoricoxib (CAS: 202409-33-4), which is a specific inhibitor of COX-2. Etoricoxib is a kind of highly selective cyclooxygenase-2 (COX-2) inhibitors developed by the Merck company. Etoricoxib was first approved for entering into market in 2002 in the UK, followed by the marketing countries and regions including the European Union, Asia, Australia and Latin America. Until the end of 2013, it has been approved for marketing in 97 countries for being widely used in treatment of osteoarthritis (OA), rheumatoid arthritis, ankylosing spondylitis, chronic low back pain, acute gouty arthritis, primary dysmenorrhea and postoperative pain, and other diseases. Etoricoxib has also entered into market in Taiwan and Hong Kong of China. It had entered into market in Chinese mainland in 2008 with the approved indications being acute gouty arthritis and OA and another indication being primary dysmenorrhea in the second half year of 2014.
-
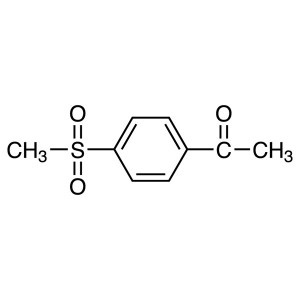
4′-(Methylsulfonyl)acetophenone CAS 10297...
-
4-(Methylsulfonyl)phenylacetic Acid CAS 90536-6...
-
4-(Methylthio)phenylacetic Acid CAS 16188-55-9 ...
-
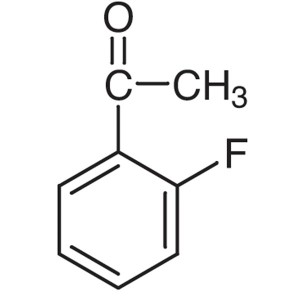
2′-Fluoroacetophenone CAS 445-27-2 Purity...
-
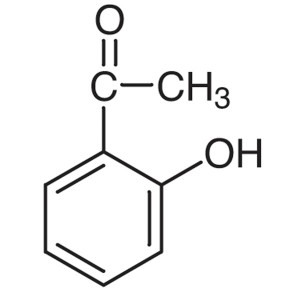
2′-Hydroxyacetophenone CAS 118-93-4 Purit...
-
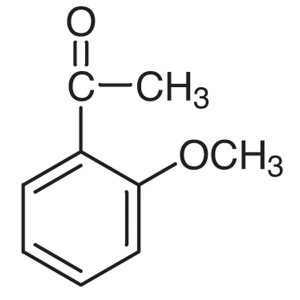
2′-Methoxyacetophenone CAS 579-74-8 Purit...
-
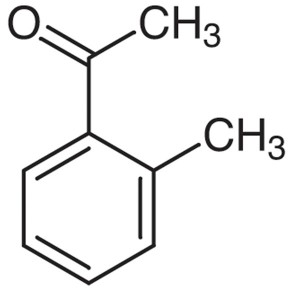
2′-Methylacetophenone CAS 577-16-2 Purity...
-
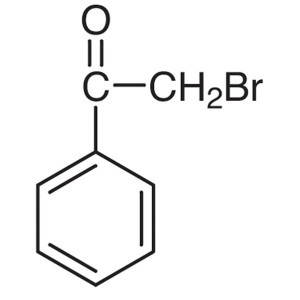
2-Bromoacetophenone CAS 70-11-1 (Phenacyl Bromi...
-
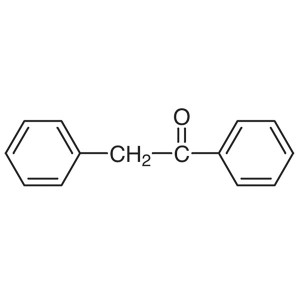
2-Phenylacetophenone (Deoxybenzoin) CAS 451-40-...
-
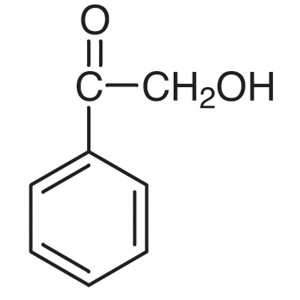
2-Hydroxyacetophenone CAS 582-24-1 Purity >99.0...
-
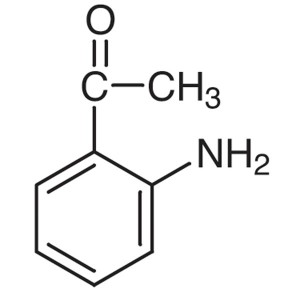
2′-Aminoacetophenone CAS 551-93-9 Purity ...
-
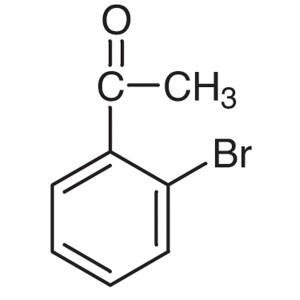
2′-Bromoacetophenone CAS 2142-69-0 Purity...
-
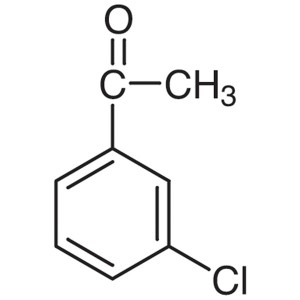
3′-Chloroacetophenone CAS 99-02-5 Purity ...
-
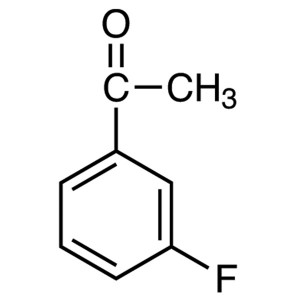
3′-Fluoroacetophenone CAS 455-36-7 Purity...
-
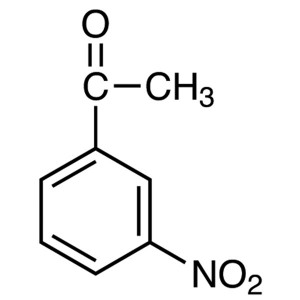
3′-Nitroacetophenone CAS 121-89-1 Purity ...